Qualitative Analysis
In simplest
terms, qualitative analysis involves the determination of what elements are
present in the portion of the sample irradiated by the electron beam. This is
accomplished by resolving and identifying the (elements giving rise to)
fluorescent characteristic x-rays emitted from the sample. Qualitative analysis
has three principal applications: phase identification, identification of
elements present for quantitative analysis, and determining proper background
offsets for quantitative analysis by WDS. Phase identification is by far the
dominant use of qualitative methods, and is an especially valuable tool for
characterizing complex, fine-grained materials. This is demonstrated in the
following example.
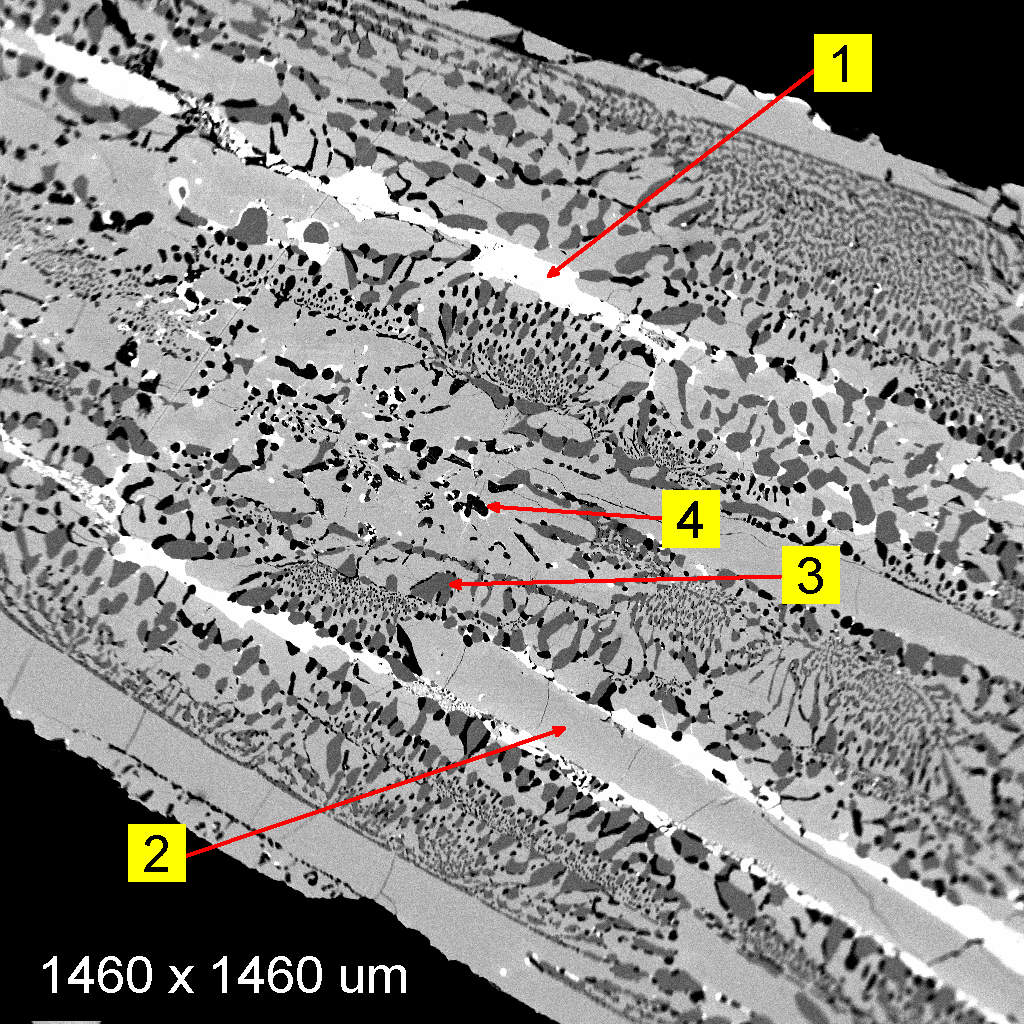
This
sample in the image at the left was suspected of being a metallic meteorite,
for which the metallic phase characteristically should be an
Fe-Ni alloy. This backscattered electron image shows morphologies consistent with
quenching (very rapid cooling) of molten material rather than the repetitive
exsolution textures (Widmanstätten patterns) that are
characteristic of (hexahedral) Ni-Fe meteorites. Qualitative analysis by EDXA
shows the individual components to be: (1) Pb-Cu-Sb
alloys; (2) Fe-As alloy; (3) Fe metal; and (4) Fe-sulfide. Hence, the textures
and compositions of the component phases identify this material as more likely
being a metallurgical slag than a metallic Ni-Fe meteorite.
Methods and Analysis
Time
As in
quantitative analysis, there are two techniques for the qualitative resolution
of fluorescent x-rays: Energy-Dispersive X-ray Analysis (EDXA) and
Wavelength-Dispersive Spectrometry (WDS) (for more information on these
methods, see the "Analyzer" section of What
is an electron microprobe?).
EDXA is the more commonly used method
for simple phase identification, because it is extremely rapid: an entire x-ray
spectrum of an unknown can be acquired and displayed in a matter of seconds.
Because the relative intensities of x-ray lines displayed in the spectrum are
proportional to the abundances of elements in the sample (especially for x-rays
arising from similar types of electron transitions), EDXA spectra allow users
to estimate the chemical stoichiometry of the sampled compound. This
application can be enhanced by semi-quantitative approaches involving rapid standardless analytical methods, which take only a few
additional seconds and can provide a reasonable estimate of elemental weight
fractions, oxide weight fractions, or atomic proportions. EDXA is especially
useful on rough samples, because the detection of x-rays is not as dependent on
beam-sample-detector geometry as is the WDS method. Therefore, EDXA can be used
very effectively with electron imaging methods to characterize loose grains or
unpolished materials. The principal limitation of EDXA as a qualitative tool
lies in detecting elements present at trace levels, which produce weak
intensities that are commonly very difficult to resolve from the background
x-radiation (the "Bremmstrählung", which is
white x-radiation produced by the deceleration of electrons of the primary
beam).
Qualitative analysis by WDS involves
scanning one or more spectrometers along a specified geometric interval, to record
the intensities of all x-ray wavelengths reaching the detector. By specifying
the range of spectrometer movement(s) and the diffraction element(s) (with
particular interplanar spacing), the analyst can
accumulate either a complete record of the x-ray spectrum or concentrate on
wavelength regions for specified elements. Although a bit more time consuming
than EDXA, rapid scans of the spectrometers can acquire a complete x-ray
spectrum in about 1 minute. The principal advantages of qualitative analysis by
WDS are superior resolution of x-ray lines and higher peak/background intensity
ratios compared to EDXA. In conjunction with options for extended counting
times and controlling the wavelength regions scanned, these advantages are
particularly important in identifying the presence of elements at trace to
minor level abundance.
Go
Back to EMPL Home Page